cvSentinel - Save time and effort!
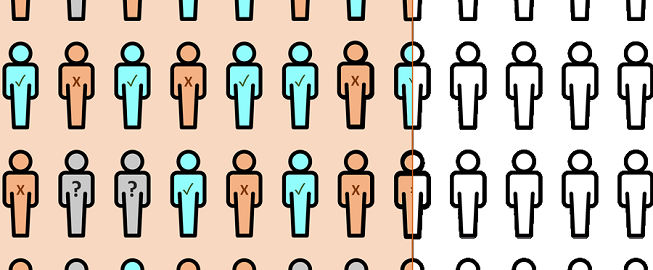
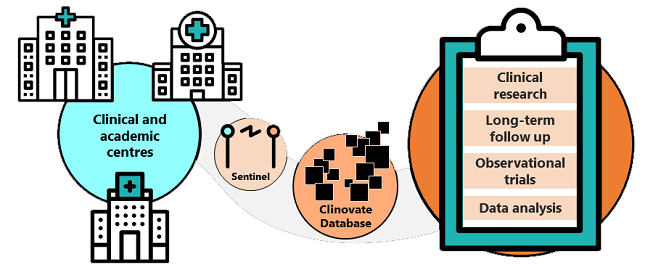
ClinHEOR is our data plattform for cvSentinel, a description data-driven collection server-client solution, that automatically transfers defined data sets of study patients from local medical documentations to our central system.
Source data with different data type, content, scope or unit are automatically prepared in such a way that they are comparable and usable for statistics within the study. These data types can be medicines (coded by the PZN pharmaceutical central number), diagnoses (coded by ICD-10 codes), laboratory values and others.
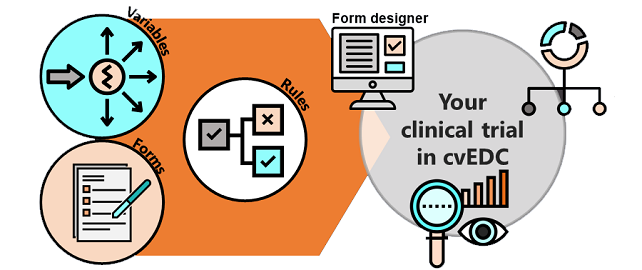
The software meets the requirements of patient data security, FDA regulations and Good Clinical Practice rules and works with the latest security features to protect data synchronisation and access. The data can be used within our cvEDC.